Introduction: The R-BAC regimen is considered among standard first-line treatments for elderly fit patients with mantle cell lymphoma (MCL). We previously reported (RBAC500 trial) a significantly inferior progression-free survival (PFS) for patients with high risk (HR) features, namely blastoid morphology and/or elevated Ki67 proliferative index, as compared to other patients, that were defined as low risk (LR). Indeed, when treated with R-BAC, LR patients had excellent outcome, albeit no maintenance therapy was delivered.
Methods: We designed a phase 2 prospective multicenter study, which enrolled patients aged ≥65 years and fit according to the geriatric CGA assessment, or age ≤64 years if not eligible to high-dose chemotherapy plus transplantation. Asymptomatic patients with non-nodal disease were excluded. At presentation patients were allocated by central review as LR or HR, depending on tumor morphology (blastoid versus others), Ki67 expression (≥30% versus others), or presence of TP53 mutation and/or deletion. Patients with any of the three risk factors were classified as HR. Patients with LR disease were treated with 6 cycles of R-BAC (rituximab 375 mg/m2 d 1; bendamustine 70 mg/m2 d 1,2; cytarabine 500 mg/m2 d 1,2,3), while HR patients received abbreviated induction with 4 R-BAC followed by consolidation (4 months, 800 mg/d), and maintenance (20 months, 400 mg/d) with venetoclax. The primary endpoint was 2-years PFS for the HR patients. The sample size was calculated with the one arm non parametric survival analysis (alpha-error 0.05, power 90%), assuming that the addition of venetoclax would improve 2-years PFS from 40% (null hypothesis) to 60%. Tumor response was assessed with Lugano criteria. All patients were analyzed by real-time quantitative PCR at baseline on peripheral blood and bone marrow samples for minimal residual disease (MRD) evaluation, and HR patients were followed up at different time points. Results of this specific analysis will be subject of future reports. This trial was registered at ClinicalTrials.gov Identifier: NCT03567876.
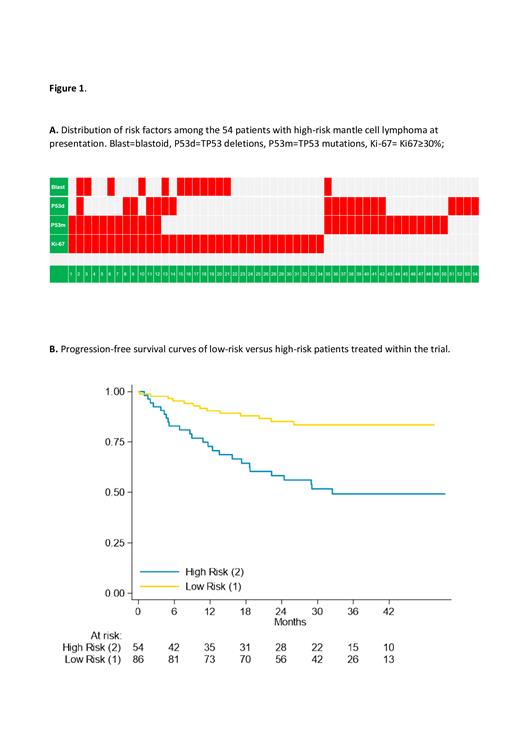
Results: Overall, 140 patients from 35 centers of the Fondazione Italiana Linfomi (FIL) were prospectively enrolled between 2018 and 2021. Of them, 54 were HR (39%). Median age was 72 (range 57-79), and 44% had elevated MIPI. LR and HR patients had similar clinical characteristics, but differed for LDH, and MIPI, both being significantly higher in the HR group. Overall, 28 (20%) patients had TP53 mutations, 19 (14%) had TP53 deletions, Ki67 was ≥30% in 34 (24%), and blastoid variant was diagnosed in 13 patients (9%, Figure 1A). Toxicity during R-BAC was in line with previous reports, while most frequent grade >=3 adverse events during venetoclax treatment consisted of neutropenia (21%), followed by skin reactions (10%). Of note, there were 5 deaths due to COVID-19 infection in patients in CR (4 LR, 1 HR). Overall response at the end of R-BAC differed between HR and LR patients (85% vs 99%, p=0.001), as was for complete response (61% vs 91%, p=0.0001). Of the 54 HR patients, 43 (80%) started venetoclax consolidation, 37 (69%) started the maintenance phase, with 26 patients (48%) completing the whole treatment per protocol. Of 10 patients that started Venetoclax in partial remission (PR) or stable disease after R-BAC, 3 converted to CR, 1 maintained PR, while 6 patients progressed during maintenance. After a median follow-up of 34 months, the 2-years PFS for the whole population was 74.9% (95% CI 66-82), and OS was 80% (95% CI 72-85). Patients with HR MCL had 2-years PFS and OS of 58% (95% CI 43-70) and 66% (95% CI 50-77), respectively, which were significantly lower than LR patients (85% and 88%, respectively, p=0.0001 for both, see Figure 1B). Predictors of PFS using Cox regression models adjusted for MIPI were blastoid morphology (Hazard Ratio 3.51), and TP53 mutation (Hazard Ratio 4.17), with Ki67, and TP53 deletions that lost their power in multivariate analysis.
Conclusions: The VR-BAC trial represents the first prospective study that stratified upfront patients with MCL to different treatments according to the risk profile. In this trial the null hypothesis (2-years PFS 40%) was rejected in HR patients, suggesting that the addition of venetoclax to R-BAC improves the performance of the induction strategy. These results point to the importance of identifying HR patients since initial diagnosis.
OffLabel Disclosure:
Visco:AbbVie, BMS, Incyte, Roche, Pfizer, Janssen, Lilly: Membership on an entity's Board of Directors or advisory committees; AbbVie, Lilly, BMS, Astra Zeneca, Servier, Incyte, Roche, Pfizer, Novartis, Gentili, Janssen, Kite-Gilead, Beigene: Honoraria, Speakers Bureau. Ferrarini:AbbVie: Research Funding. Zilioli:Novartis: Membership on an entity's Board of Directors or advisory committees; Janssen: Other: travel expenses, Speakers Bureau; Lilly: Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: travel expenses, Speakers Bureau; Servier: Speakers Bureau; Roche: Consultancy, Other: travel expenses; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; MSD: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Speakers Bureau. Re:Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees; Italfarmaco: Membership on an entity's Board of Directors or advisory committees. Corradini:SOBI: Other: Honoraria (Consulting, advisory role, or lecturer); Daiichi Sankyo: Other: Honoraria (Consulting, advisory role, or lecturer); Nerviano Medical Science: Other: Honoraria (Consulting, advisory role, or lecturer); Kyowa Kirin: Other: Honoraria (Consulting, advisory role, or lecturer); Novartis: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Pfizer: Other: Honoraria (Consulting, advisory role, or lecturer); Sanofi: Other: Honoraria (Consulting, advisory role, or lecturer); Roche: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Janssen: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Amgen: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; ADC Theraputics (DSMB): Other: Honoraria (Consulting, advisory role, or lecturer); Incyte: Other: Honoraria (Consulting, advisory role, or lecturer); Gilead/Kite: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Celgene: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; AbbVie: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; Takeda: Other: Honoraria (Consulting, advisory role, or lecturer), Travel and accomodations; GlaxoSmithKline: Other: Honoraria (Consulting, advisory role, or lecturer); BeiGene: Honoraria; Bristol Myers Squibb: Other: Travel and accomodations. Hohaus:Takeda: Honoraria, Research Funding; Roche: Research Funding; Kiowa Kirin: Honoraria; MDS: Honoraria; Janssen: Speakers Bureau; Gentili: Speakers Bureau; Sanofi: Speakers Bureau; Incyte: Speakers Bureau. Musuraca:Takeda: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Cavallo:Roche: Honoraria, Speakers Bureau; Takeda: Research Funding; Astra Zeneca: Research Funding; Beigene: Research Funding. Di Rocco:Incyte: Speakers Bureau; Gilead: Honoraria, Speakers Bureau; Janssen: Honoraria; Abbvie: Honoraria; Takeda: Speakers Bureau; Novartis: Speakers Bureau; Roche: Honoraria, Speakers Bureau. Arcari:Janssen, Abbvie, Takeda, Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Merli:Gilead: Other: advisory board; Roche: Other: advisory board; Novartis: Other: advisory board; Takeda: Other: advisory board; Incyte: Other: advisory board; Janssen: Other: advisory board; MSD: Other: advisory board. Gini:Takeda: Consultancy; Gentili: Consultancy; Incyte: Consultancy; Roche: Consultancy. Tani:Abbvie, Jansen-Cilag, Incyte: Membership on an entity's Board of Directors or advisory committees. Ferreri:Adienne: Speakers Bureau; Gilead, Incyte, Novartis, PentixaPharm, Roche: Consultancy; ADC Therapeutics, Amgen, BeiGene, BMS, Genmab, Gilead, Hutchison Medipharma, Novartis, Pharmacyclics, PentixaPharm, Pfizer, Roche: Research Funding; Ospedale San Raffaele srl.: Patents & Royalties. Santoro:Bayer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Lilly: Speakers Bureau; Sandoz: Speakers Bureau; Eisai: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Speakers Bureau; Arqule: Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Speakers Bureau; Amgen: Speakers Bureau; AbbVie: Speakers Bureau; Roche: Speakers Bureau; BMS (Bristol Myers Squibb): Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Speakers Bureau; Incyte: Consultancy; Sanofi: Consultancy; MSD (Merck Sharp & Dohme): Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Patti:MSD: Research Funding. Ladetto:Novartis: Honoraria. Zinzani:SANDOZ: Membership on an entity's Board of Directors or advisory committees; JANSSEN-CILAG: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SECURA BIO: Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SERVIER: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASTRAZENECA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TAKEDA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC THERAPEUTICS: Membership on an entity's Board of Directors or advisory committees; CELLTRION: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GILEAD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSAPHARMA: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; INCYTE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ROCHE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; NOVARTIS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; KYOWA KIRIN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BEIGENE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Pileri:Stemline: Speakers Bureau; Lilly: Speakers Bureau; Diatech: Consultancy; Roche: Speakers Bureau; Beigene: Speakers Bureau; Nanostring: Speakers Bureau; Celgene: Speakers Bureau.
Venetoclax monotherapy following response to chemo-immmunotherapy

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal